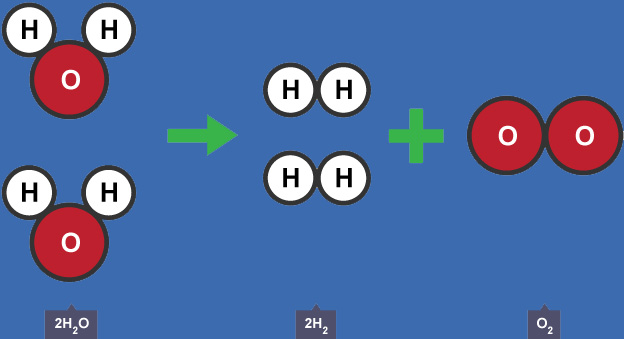
The electrolysis of the water process splits the water molecules (H2O) into Hydrogen (H2) and Oxygen (O2) molecules according to the following expression:
Water => Hydrogen + Oxygen
2 * H2O(liquid) => 2 * H2(gas) + O2(gas)
This process is driven by a redox reaction (reduction–oxidation reaction) that, to simplify the concept, we can describe with the following two half reactions:
Oxidation Reaction
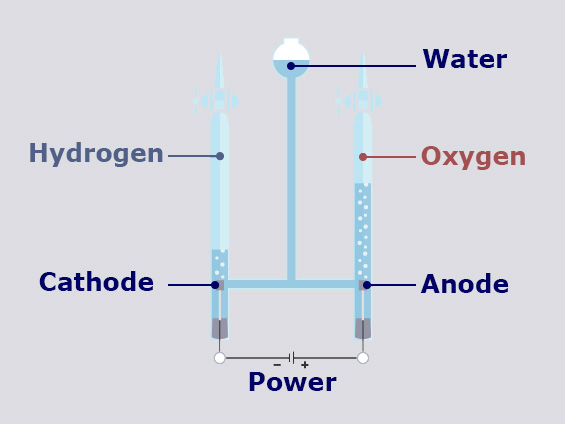
At the positively charged electrode (anode) an oxidation reaction occurs, where 2 molecules of water (H20) split in:
- 1 Molecule of Oxygen (O2) in gaseous form.
- 4 Ions of Hydrogen (H+).
- 4 Free electrons (e–).
The 4 Free electrons (e–) go to the anode electrode (they split from the water because they have been attracted by the positive charges present at the anode electrode), the gaseous oxygen (O2 ) get out of the water and it is one of the components produced by this reaction, the 4 Ions of Hydrogen (H+) stay in the water.
This reaction can be represented by the following expression:
Oxidation at anode: 2 * H2O(liquid) => O2(gas) + 4 * H+(Aqueous Solution) + 4e−
Reduction Reaction
At the negative charged electrode (cathode) a reduction reaction occurs, where 4 Ions of Hydrogen (H+) attract 4 Free electrons (e–) from the cathode electrode and go to form 2 molecules of Hydrogen (H2).
This reaction can be represented by the following expression:
Reduction at cathode: 4 * H+(Aqueous Solution) + 4e– => 2 * H2(gas)
Redox Reaction
So the whole process can be represented by the following expressions:
Oxidation at anode: 2 * H2O(liquid) => O2(gas) + 4 * H+(Aqueous Solution) + 4e−
Reduction at cathode: 4 * H+(Aqueous Solution) + 4e– => 2 * H2(gas)
that combined can give the final expression:
Overall reaction: 2 * H2O(liquid) => 2 * H2(gas) + O2(gas)

